Science and Technology
In News: The national drug regulator, DGCI, has given the green signal to the country’s first intra-nasal Covid vaccine for emergency use in adults – iNCOVACC.
- Manufactured by Bharat Biotech, the company behind Covaxin, in partnership with Washington University-St Louis, and partly funded by the Department of Biotechnology’s Covid Suraksha programme
- The new vaccine has been approved for primary immunisation — it can be administered only to the unimmunised.
- Those who have already received the first and second doses of other vaccines will not be eligible to get iNCOVACC as the “precaution” third dose.
Source: Bharat Biotech
The Administration
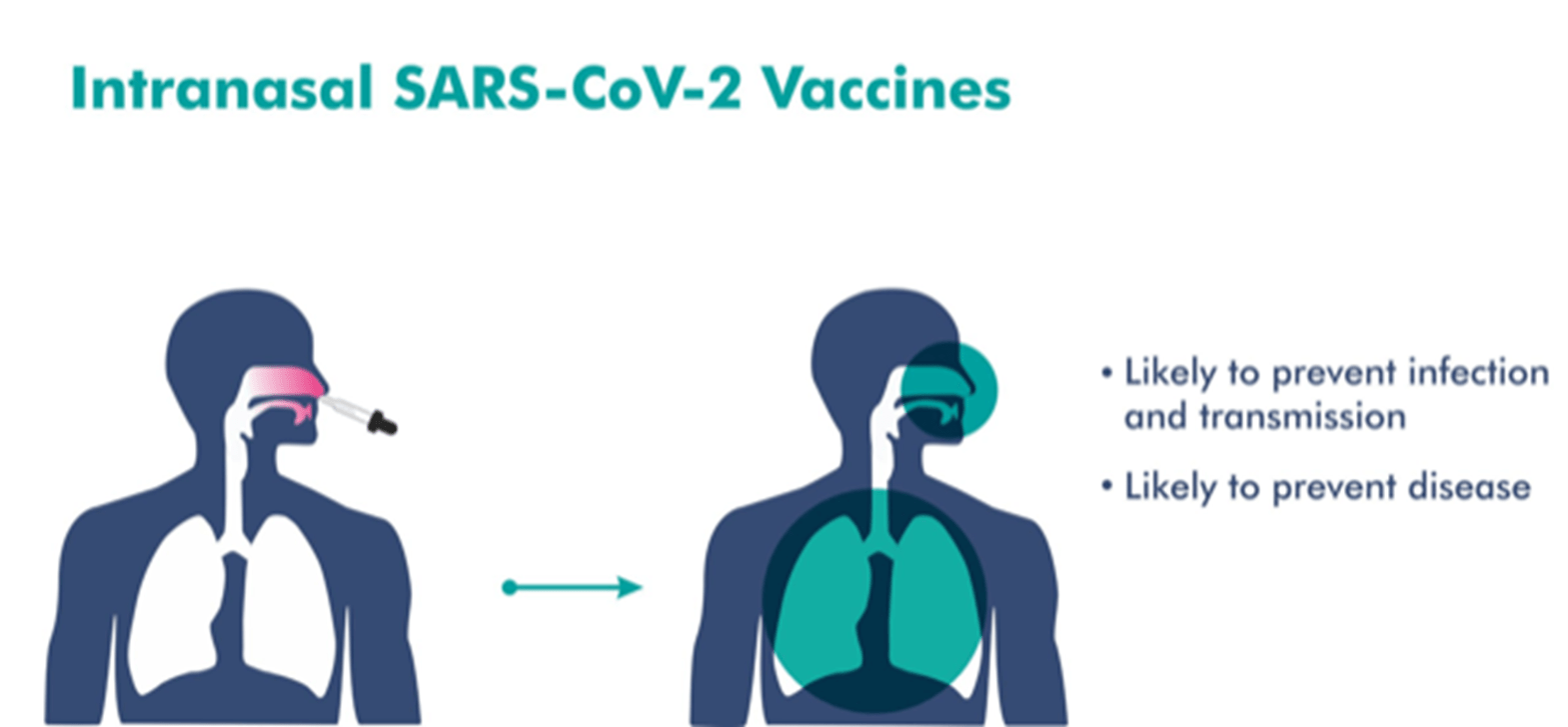
- Will be delivered through the nasal route.
- This would potentially trigger an immune response in the mucosal membrane.
- It has been designed to not only protect against infection but also reduce transmission of the virus.
- May produce local antibodies in the upper respiratory tract, which may provide the potential to reduce infection and transmission.
- The vaccine uses a modified chimpanzee adenovirus, which cannot replicate in the body, to carry the Covid spike protein to induce immunity.
Benefits of intranasal vaccine
- Promises to be more effective, since it is expected to generate immune responses at the site of infection (respiratory mucosa)
- Non-invasive, Needle-free.
- Ease of administration – does not require trained health care workers.
- Elimination of needle-associated risks (injuries and infections).
- High compliance (Ideally suits for children’s and adults).
- Scalable manufacturing – able to meet global demand. It can produce 100 million doses a month.
India’s Progress Card
- India has, so far, administered a total of 213 crore vaccine doses, of which 102 crore are first doses
- Nearly 98 per cent of adults in India had received at least one dose by the third week of July
- Currently, Covishield, Covaxin and Corbevax are part of the Government’s Covid immunisation drive while vaccines like Covovax and Sputnik are available at private centres.
About Drugs Controller General of India
- Drugs Controller General of India is the head of department of the Central Drugs Standard Control Organization of the Government of India.
- Responsible for approval of licences of specified categories of drugs such as blood and blood products, IV fluids, vaccines, and sera in India
- DCGI also sets standards for manufacturing, sales, import, and distribution of drugs in India.
- Comes under the Ministry of Health & Family Welfare.
- DCGI lays down the standard and quality of manufacturing, selling, import and distribution of drugs in India.
- Acting as appellate authority in case of any dispute regarding the quality of drugs
- Preparation and maintenance of national reference standard
- To bring about the uniformity in the enforcement of the Drugs and Cosmetics Act.
- DCGI also act as Central Licensing Authority (CLA) for the medical devices which fall under the purview of Medical Device Rules 2017
Source: The Indian Express
Previous Year Question
Q.1) In the context of vaccines manufactured to prevent COVID-19 pandemic, consider the following statements: (2022)
- The Serum Institute of India produced COVID-19 vaccine named Covishield using mRNA platform.
- Sputnik V vaccine is manufactured using vector based platform.
- COVAXIN is an inactivated pathogen based vaccine.
Which of the statements given above are correct?
- 1 and 2 only
- 2 and 3 only
- 1 and 3 only
- 1, 2 and 3











