Science and Technology
In News: Rajya Sabha MP from the Nationalist Congress Party Fauzia Khan on Friday raised concerns over the benefits of the National Policy of Rare Diseases (NPRD) not reaching any patient with rare diseases even after several months since its introduction.
Context:
- The Union Ministry of Health and Family Welfare notified the NPRD in March 2021.
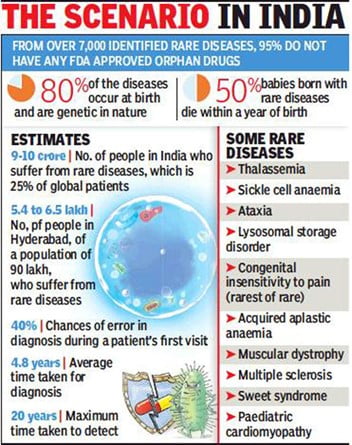
- An estimated 5000 to 8000 rare diseases have been identified worldwide, affecting approximately 6 to 8% of the population.
- Individual rare diseases affect few people, but cumulatively have a major impact on public health.
What are Rare diseases:
- A rare disease is any disease that affects a small percentage of the population such as fewer than 200,000 people across a broad range of possible disorders.
- These rare diseases are majorly thought to be genetic and are passed on from one generation to the next.
- In India, Haemophilia, Thalassemia, Sickle cell anaemia and Primary Immuno Deficiency in children, auto-immune diseases, Lysosomal storage disorders such as Pompe disease and Gaucher’s disease are in the rare diseases list.
National Policy of Rare Diseases (NPRD) 2021
- Objectives – promotion of research and development for diagnosis and treatment of rare diseases.
- Promotion of local development and manufacture of drugs and creation of conducive environment for indigenous manufacturing of drugs for rare diseases at affordable prices.
- The rare diseases have been identified and categorized into 3 groups.
- Group 1: Disorders amenable to one-time curative treatment.
- Group-2: Diseases requiring long term/lifelong treatment having relatively lower cost of treatment and benefit has been documented in literature and annual or more frequent surveillance is required.
- Group 3:- Diseases for which definitive treatment is available but challenges are to make optimal patient selection for benefit, very high cost and lifelong therapy.
- Eight (08) Centres of Excellence (CoEs) have been identified for diagnosis, prevention and treatment of rare diseases.
- Five Nidan Kendras have been set up for genetic testing and counselling services.
Challenges in India:
- Lack of treatment: About 95% rare diseases have no approved treatment and less than 1 in 10 patients receive disease specific treatment.
- Loss of lives due to delayed and misdiagnoses, limited access to resources, and absence of specific therapies often preclude patients from receiving proper, timely care.
- Impact on children: Children are disproportionately affected by these diseases as compared to adults
- 50 percent of new cases are observed to be in children, out of which 35 percent of children die before the age of one year, 10 percent die between the ages of 1 to 5 years, and 12 percent between the ages of 5 to 15 years. (Ministry of Health and Family Welfare 2017)
- High cost: The cost of treatment of rare diseases may vary from INR 10 lakhs to INR 1 crore on an annual basis.
- Issues in policy design: India does not have its standard definition for rare disease and neither does sufficient data on prevalence exists.
- The Government of India launched the Indian Rare Disease Registry only in April 2017.
- Only 450 rare diseases have been recorded in the registry as per data available from tertiary hospitals
- Unending delay and lack of urgency in policy implementation
- Eg lack of will of Centres of Excellence (CoE), designated as per the NPRD policy, has endangered the survival prospects of 415 patients, largely children, diagnosed with rare diseases.
- Majority of these patients have been diagnosed with Gaucher disease, for which therapy approved by Drug Controller General of India is available for many years.
- Lack of governance
- In NPRD policy, many CoEs were yet to seek financial support as per the policy for treating the patients.
Suggestions:
- The amount of 20 lakh sanctioned under the Rashtriya Arogya Nidhi, although appreciable but is barely enough to cover the costs of treatment.
- The Central Government recently informed the Delhi High Court that a digital platform has been made operational for crowdfunding of treatment and medicines for rare disease.
- Support from foundations, NGO’s and crowdfunding-led initiatives is extremely helpful, however, it will not be accessible to all and is only a stop-gap solution.
- As of August 2019, Takeda Pharmaceutical Company’s programme has covered 199 patients from 13 countries including India.
- Sustained medical and financial support to the patient from Government.
- Robust and inclusive policy in consultation with the State Governments.
- Public health concern is addressed sustainably to provide respite to thousands of victims of rare diseases and their families in India.
Way forward
- The private initiatives are leading the war, but without support from the Government, such solutions are not sustainable.
- Strength of policy making is integral to the strength of the government as a whole, and that of the country at large.
Source The Hindu














