IASbaba Prelims 60 Days Plan, Rapid Revision Series (RaRe)
Archives
Hello Friends
The 60 Days Rapid Revision (RaRe) Series is IASbaba’s Flagship Initiative recommended by Toppers and loved by the aspirants’ community every year.
It is the most comprehensive program which will help you complete the syllabus, revise and practice tests on a daily basis. The Programme on a daily basis includes
Daily Prelims MCQs from Static (Monday – Saturday)
- Daily Static Quiz will cover all the topics of static subjects – Polity, History, Geography, Economics, Environment and Science and technology.
- 20 questions will be posted daily and these questions are framed from the topics mentioned in the schedule.
- It will ensure timely and streamlined revision of your static subjects.
Daily Current Affairs MCQs (Monday – Saturday)
- Daily 5 Current Affairs questions, based on sources like ‘The Hindu’, ‘Indian Express’ and ‘PIB’, would be published from Monday to Saturday according to the schedule.
Daily CSAT Quiz (Monday – Friday)
- CSAT has been an Achilles heel for many aspirants.
- Daily 5 CSAT Questions will be published.
Note – Daily Test of 20 static questions, 5 current affairs, and 5 CSAT questions. (30 Prelims Questions) in QUIZ FORMAT will be updated on a daily basis.
To Know More about 60 Days Rapid Revision (RaRe) Series – CLICK HERE
60 Day Rapid Revision (RaRe) Series Schedule – CLICK HERE
60 Day Rapid Revision (RaRe) Series Questions & Solutions DAY 47– CLICK HERE
Important Note
- Comment your Scores in the Comment Section. This will keep you accountable, responsible and sincere in days to come.
- It will help us come out with the Cut-Off on a Daily Basis.
- Let us know if you enjoyed today’s test 🙂
- You can post your comments in the given format
- (1) Your Score
- (2) Matrix Meter
- (3) New Learning from the Test
Test-summary
0 of 30 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
Information
The following Test is based on the syllabus of 60 Days Plan-2023 for UPSC IAS Prelims 2022.
To view Solutions, follow these instructions:
- Click on – ‘Start Test’ button
- Solve Questions
- Click on ‘Test Summary’ button
- Click on ‘Finish Test’ button
- Now click on ‘View Questions’ button – here you will see solutions and links.
You have already completed the test before. Hence you can not start it again.
Test is loading...
You must sign in or sign up to start the test.
You have to finish following test, to start this test:
Results
0 of 30 questions answered correctly
Your time:
Time has elapsed
You have scored 0 points out of 0 points, (0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- Answered
- Review
-
Question 1 of 30
1. Question
Consider the following statements about atoms
- They are the smallest unit of matterand combine to form
- The nucleusof the atom contains both protons and electrons.
- Neutronsare the heaviest while electrons are the smallest of the subatomic particles.
Which of the following statements is/are correct?
Correct
Solution: (c)
Explanation:
- Atomsare the smallest unit of matter (something which can be physically touched) that retains all the chemical properties of an element.
- They combine to form molecules(which in turn form solids, gases, and liquids). Hence statement 1 is correct.
- Atoms are made of three basic particles. These are electrons, protons, and neutrons– they are collectively referred to as subatomic particles.
- The nucleusof the atom contains both protons (which are positively charged particles) and neutrons (which have no charge).
- These protons and neutrons are collectively called nucleonsand are bound together by a force called the residual strong force. Hence statement 2 is incorrect.
- Neutronsare the heaviest subatomic particle and weigh 1839 times more than an electron. They possess no charge at all.
- Protonspossess a positive charge and are 1836 times heavier than an electron.
- Electronsare the smallest of the subatomic particles (they are too small to be measured using techniques currently available to scientists) and have a negative
- They are bound to the nucleusdue to their opposite electrical charges. Hence statement 3 is correct.
Incorrect
Solution: (c)
Explanation:
- Atomsare the smallest unit of matter (something which can be physically touched) that retains all the chemical properties of an element.
- They combine to form molecules(which in turn form solids, gases, and liquids). Hence statement 1 is correct.
- Atoms are made of three basic particles. These are electrons, protons, and neutrons– they are collectively referred to as subatomic particles.
- The nucleusof the atom contains both protons (which are positively charged particles) and neutrons (which have no charge).
- These protons and neutrons are collectively called nucleonsand are bound together by a force called the residual strong force. Hence statement 2 is incorrect.
- Neutronsare the heaviest subatomic particle and weigh 1839 times more than an electron. They possess no charge at all.
- Protonspossess a positive charge and are 1836 times heavier than an electron.
- Electronsare the smallest of the subatomic particles (they are too small to be measured using techniques currently available to scientists) and have a negative
- They are bound to the nucleusdue to their opposite electrical charges. Hence statement 3 is correct.
-
Question 2 of 30
2. Question
Consider the following statements
- Atomic mass number is the total number of neutrons and protons in the atom’s nucleus.
- The atomic number is the total number of protons in the atom’s nucleus.
Choose the correct code:
Correct
Solution: (c)
Explanation:
- The atomic mass number, also known as the mass number, represents the total number of protons and neutrons in the nucleus of an atom. Protons are positively charged particles, while neutrons are neutrally charged particles. Together, protons and neutrons make up the majority of an atom’s mass. Electrons, which have a much smaller mass compared to protons and neutrons, orbit around the nucleus.
- For example, let’s consider the element carbon. The atomic number of carbon is 6, indicating it has 6 protons. Carbon has several isotopes, including carbon-12, carbon-13, and carbon-14. The number after the element name represents the atomic mass number for each isotope. Carbon-12 has 6 protons and 6 neutrons, carbon-13 has 6 protons and 7 neutrons, and carbon-14 has 6 protons and 8 neutrons. In all cases, the sum of protons and neutrons gives us the atomic mass number.Hence statement 1 is correct
- The atomic number of an atom represents the total number of protons in its nucleus. It is a fundamental property of an element and helps uniquely identify it. Each element has a distinct atomic number. The periodic table is arranged based on increasing atomic numbers.
- The number of protons determines the element’s chemical properties and its position in the periodic table. For example, hydrogen has an atomic number of 1, indicating it has one proton in its nucleus. Helium has an atomic number of 2, indicating it has two protons. This pattern continues throughout the periodic table.
- It’s important to note that the atomic number also determines the number of electrons in a neutral atom. In an electrically neutral atom, the number of protons equals the number of electrons. Electrons occupy the electron shells surrounding the nucleus, balancing the positive charge of protons. Hence statement 2 is correct
Incorrect
Solution: (c)
Explanation:
- The atomic mass number, also known as the mass number, represents the total number of protons and neutrons in the nucleus of an atom. Protons are positively charged particles, while neutrons are neutrally charged particles. Together, protons and neutrons make up the majority of an atom’s mass. Electrons, which have a much smaller mass compared to protons and neutrons, orbit around the nucleus.
- For example, let’s consider the element carbon. The atomic number of carbon is 6, indicating it has 6 protons. Carbon has several isotopes, including carbon-12, carbon-13, and carbon-14. The number after the element name represents the atomic mass number for each isotope. Carbon-12 has 6 protons and 6 neutrons, carbon-13 has 6 protons and 7 neutrons, and carbon-14 has 6 protons and 8 neutrons. In all cases, the sum of protons and neutrons gives us the atomic mass number.Hence statement 1 is correct
- The atomic number of an atom represents the total number of protons in its nucleus. It is a fundamental property of an element and helps uniquely identify it. Each element has a distinct atomic number. The periodic table is arranged based on increasing atomic numbers.
- The number of protons determines the element’s chemical properties and its position in the periodic table. For example, hydrogen has an atomic number of 1, indicating it has one proton in its nucleus. Helium has an atomic number of 2, indicating it has two protons. This pattern continues throughout the periodic table.
- It’s important to note that the atomic number also determines the number of electrons in a neutral atom. In an electrically neutral atom, the number of protons equals the number of electrons. Electrons occupy the electron shells surrounding the nucleus, balancing the positive charge of protons. Hence statement 2 is correct
-
Question 3 of 30
3. Question
Consider the following statements
- Molecules are formed by two or more atoms that are bonded by chemical bondings.
- Table salt, ammonia, methane, etc. are examples of molecules.
- Compounds are formed by two or more elements where the elements are mixed in fixed ratios.
- Ozone and nitrogen are examples of compounds.
Choose the correct code:
Correct
Solution: (a)
Explanation:
- Molecules are formed by two or more atoms that are bonded by chemical bondings.
- Structurally, molecules are composed of two or more atoms held by a very strong force.
- Not all molecules can be called compounds. Hence statement 1 is correct.
- It is not possible to see a molecule with one’s naked eye because they are formed at atomic levels.
- Ozone, a molecule of oxygen, and nitrogen are examples of molecules. Hence statement 4 is incorrect.
- Compounds are formed by two or more elements where the elements are mixed in fixed ratios.
- Every compound is under the category of the matter when they are in absolute shape.Hence statement 3 is correct.
- Compounds can be viewed with one’s naked eyes very easily.
- Table salt, ammonia, methane, etc. are examples of compounds. Hence statement 2 is incorrect.
Incorrect
Solution: (a)
Explanation:
- Molecules are formed by two or more atoms that are bonded by chemical bondings.
- Structurally, molecules are composed of two or more atoms held by a very strong force.
- Not all molecules can be called compounds. Hence statement 1 is correct.
- It is not possible to see a molecule with one’s naked eye because they are formed at atomic levels.
- Ozone, a molecule of oxygen, and nitrogen are examples of molecules. Hence statement 4 is incorrect.
- Compounds are formed by two or more elements where the elements are mixed in fixed ratios.
- Every compound is under the category of the matter when they are in absolute shape.Hence statement 3 is correct.
- Compounds can be viewed with one’s naked eyes very easily.
- Table salt, ammonia, methane, etc. are examples of compounds. Hence statement 2 is incorrect.
-
Question 4 of 30
4. Question
Consider the following statements about the Bose-Einstein Condensates (BECs)
- Its existence was predicted by Albert Einstein and Satyendra Nath Bose.
- At this point, the atoms become a single entity with quantum properties.
- It contains vital clues to mysterious phenomena such as dark energy.
Choose the correct code:
Correct
Solution: (d)
Explanation:
- The existence of Bose-Einstein condensates (BEC) was predicted by Indian mathematician Satyendra Nath Bose and Albert Einstein almost a century ago. Hence statement 1 is correct.
- Bose-Einstein Condensates (BEC) are formed when the atoms of certain elements are cooled to near absolute zero (0 K or – 273.15°C).
- At this point, atoms become a single entity with quantum property, whereas each particle also functions as a wave of matter. Hence statement 2 is correct.
- Scientists have believed that BECs contain vital clues to mysterious phenomena such as dark energy which is unknown energy thought to be behind the Universe’s accelerating expansion.
- These are extremely fragile and the slightest interaction with the external world is enough to warm them past their condensation threshold.
- Because of this condition, it becomes nearly impossible for scientists to study BECs on Earth as gravity interferes with the magnetic field required to hold them in place for observation.
- BECs in terrestrial labs generally last a handful of milliseconds before dissipating while aboard ISS, those lasted more than a second.
- Studying BECs in microgravity has opened up a host of opportunities.
- Applications range from:
- Studying gravitational waves
- Spacecraft navigation
- Searches for dark energy
- Tests of general relativity
- Prospecting for subsurface minerals on the moon and other planetary bodies
Hence statement 3 is correct.
Incorrect
Solution: (d)
Explanation:
- The existence of Bose-Einstein condensates (BEC) was predicted by Indian mathematician Satyendra Nath Bose and Albert Einstein almost a century ago. Hence statement 1 is correct.
- Bose-Einstein Condensates (BEC) are formed when the atoms of certain elements are cooled to near absolute zero (0 K or – 273.15°C).
- At this point, atoms become a single entity with quantum property, whereas each particle also functions as a wave of matter. Hence statement 2 is correct.
- Scientists have believed that BECs contain vital clues to mysterious phenomena such as dark energy which is unknown energy thought to be behind the Universe’s accelerating expansion.
- These are extremely fragile and the slightest interaction with the external world is enough to warm them past their condensation threshold.
- Because of this condition, it becomes nearly impossible for scientists to study BECs on Earth as gravity interferes with the magnetic field required to hold them in place for observation.
- BECs in terrestrial labs generally last a handful of milliseconds before dissipating while aboard ISS, those lasted more than a second.
- Studying BECs in microgravity has opened up a host of opportunities.
- Applications range from:
- Studying gravitational waves
- Spacecraft navigation
- Searches for dark energy
- Tests of general relativity
- Prospecting for subsurface minerals on the moon and other planetary bodies
Hence statement 3 is correct.
-
Question 5 of 30
5. Question
Consider the following statements about the Rubidium
- It is a very soft, silvery-white metal in the alkali metal group.
- It is used in fireworks to give them a purple colour.
- It can be stored under atmospheric oxygen.
Which of the following statements is/are correct?
Correct
Solution: (a)
Explanation:
- Rubidium is a chemical element with the symbol Rb and atomic number 37.
- It is a very soft, silvery-white metal in the alkali metal group. Hence statement 1 is correct.
- It is used in fireworks to give them a purple colour.
- It has also been considered for use in a thermoelectric generator.
- Vaporized 87Rb is one of the most commonly used atomic species employed for laser cooling and Bose-Einstein condensation. Hence statement 2 is correct.
- It cannot be stored under atmospheric oxygen, as a highly exothermic reaction will ensue, sometimes even resulting in the metal catching fire. Hence statement 3 is incorrect.
Incorrect
Solution: (a)
Explanation:
- Rubidium is a chemical element with the symbol Rb and atomic number 37.
- It is a very soft, silvery-white metal in the alkali metal group. Hence statement 1 is correct.
- It is used in fireworks to give them a purple colour.
- It has also been considered for use in a thermoelectric generator.
- Vaporized 87Rb is one of the most commonly used atomic species employed for laser cooling and Bose-Einstein condensation. Hence statement 2 is correct.
- It cannot be stored under atmospheric oxygen, as a highly exothermic reaction will ensue, sometimes even resulting in the metal catching fire. Hence statement 3 is incorrect.
-
Question 6 of 30
6. Question
Which of the following is the rarest element on earth?
Correct
Solution: (b)
Explanation:
- Halogens are any of the elements fluorine, chlorine, bromine, iodine, and astatine, occupying group VIIA (17) of the periodic table.
- They are reactive non-metallic elements that form strongly acidic compounds with hydrogen from which simple salts can be made.
- Astatine is a radioactive chemical element that has the symbol At. The astatine atomic number is 85.
- It is amongst the rarest naturally occurring element from the crust of the Earth and occurs only when there is a decay of several heavier elements. There are about 28 g of it in the Earth’s crust.
- Its chemical properties are known to be much similar to that of iodine.
- It has not been much researched because all its isotopes have shorter half-lives.
- Since astatine behaves much similarly to iodine, it gets secreted in the human thyroid gland. Therefore, it is used in the treatment of diseases related to the thyroid.
- The isotope of astatine called Astatine-211 is used in the procedure of radiotherapy. It is also utilized for the treatment of cancer since it is said to destroy cancer-causing cells.
Hence option b is correct.
Incorrect
Solution: (b)
Explanation:
- Halogens are any of the elements fluorine, chlorine, bromine, iodine, and astatine, occupying group VIIA (17) of the periodic table.
- They are reactive non-metallic elements that form strongly acidic compounds with hydrogen from which simple salts can be made.
- Astatine is a radioactive chemical element that has the symbol At. The astatine atomic number is 85.
- It is amongst the rarest naturally occurring element from the crust of the Earth and occurs only when there is a decay of several heavier elements. There are about 28 g of it in the Earth’s crust.
- Its chemical properties are known to be much similar to that of iodine.
- It has not been much researched because all its isotopes have shorter half-lives.
- Since astatine behaves much similarly to iodine, it gets secreted in the human thyroid gland. Therefore, it is used in the treatment of diseases related to the thyroid.
- The isotope of astatine called Astatine-211 is used in the procedure of radiotherapy. It is also utilized for the treatment of cancer since it is said to destroy cancer-causing cells.
Hence option b is correct.
-
Question 7 of 30
7. Question
Consider the following statements
- Metals generally occur as hard solid substances whereas non-metals occur in all three forms of matter.
- Metals are non-malleable and non-ductile whereas non-metals are malleable and ductile.
- Metals show the sonorous property whereas non-metals do not show the sonorous property.
Which of the following statements is/are correct?
Correct
Solution: (c)
Explanation:
- Metals occur as hard solid substances whereas non-metals occur in all three forms of matter.
- Metals are good conductors of heat and electricity whereas non-metals are poor conductors of heat and electricity with the exception of graphite which is a good conductor of heat and electricity. Hence statement 1 is correct.
- Metals are the elements that conduct heat and electricity and are malleable and ductile.
- Examples: Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu), Gold (Au), Platinum (Pt), Lead (Pb), Potassium (K), Sodium (Na), Calcium (Ca) and Magnesium (Mg), etc.
- Metals are the elements that form positive ions by losing electrons. Thus, metals are known as Electropositive Elements.
- Non-metals are the elements that do not conduct electricity and are neither malleable nor ductile.
Examples: Carbon (C), Sulphur (S), Phosphorous (P), Silicon (Si), Hydrogen (H), Oxygen (O), Nitrogen (N), Chlorine (Cl), Bromine (Br), Neon (Ne) and Argon (Ar), etc.
Non-metals are the elements that form negative ions by gaining an electron. Thus, non-metals are also known as Electronegative Elements. - Metals are malleable and ductile whereas non-metals are non-malleable and non-ductile. Hence statement 2 is incorrect.
- Metals produce a ringing sound on striking which is called their sonorous property.
- Metals show the sonorous property whereas non-metals do not show the sonorous property. Hence statement 3 is correct.
Incorrect
Solution: (c)
Explanation:
- Metals occur as hard solid substances whereas non-metals occur in all three forms of matter.
- Metals are good conductors of heat and electricity whereas non-metals are poor conductors of heat and electricity with the exception of graphite which is a good conductor of heat and electricity. Hence statement 1 is correct.
- Metals are the elements that conduct heat and electricity and are malleable and ductile.
- Examples: Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu), Gold (Au), Platinum (Pt), Lead (Pb), Potassium (K), Sodium (Na), Calcium (Ca) and Magnesium (Mg), etc.
- Metals are the elements that form positive ions by losing electrons. Thus, metals are known as Electropositive Elements.
- Non-metals are the elements that do not conduct electricity and are neither malleable nor ductile.
Examples: Carbon (C), Sulphur (S), Phosphorous (P), Silicon (Si), Hydrogen (H), Oxygen (O), Nitrogen (N), Chlorine (Cl), Bromine (Br), Neon (Ne) and Argon (Ar), etc.
Non-metals are the elements that form negative ions by gaining an electron. Thus, non-metals are also known as Electronegative Elements. - Metals are malleable and ductile whereas non-metals are non-malleable and non-ductile. Hence statement 2 is incorrect.
- Metals produce a ringing sound on striking which is called their sonorous property.
- Metals show the sonorous property whereas non-metals do not show the sonorous property. Hence statement 3 is correct.
-
Question 8 of 30
8. Question
Consider the following statements about Ionic compounds
- They have low melting and boiling points.
- They are solid and brittle.
- They are generally insoluble in organic solvents.
Which of the following statements is/are correct?
Correct
Solution: (b)
Explanation:
- Ionic bonds are formed because of the transfer of electrons from the metal to the non-metal.
- In this course, metals get a positive charge because of the transfer of electrons and non-metal gets a negative charge because of acceptance of electrons. In other words, the bond formed between positive and negative ions is called Ionic Bond.
Since, a compound is electrically neutral, to form an ionic compound, negative and positive both ions must be combined. - The properties of an Ionic compound are:
- Ionic compounds have high melting and boiling points because the force of attraction between ions of ionic compounds is very strong. Hence statement 1 is incorrect.
- Ionic compounds are solid. Ionic bond has a greater force of attraction because of which ions attract each other strongly. This makes ionic compounds solid.
- Ionic compounds are brittle. Hence statement 2 is correct.
- Ionic compounds generally dissolve in water.
- Ionic compounds are generally insoluble in organic solvents; like kerosene, petrol, etc. Hence statement 3 is correct.
- Ionic compounds do not conduct electricity in the solid state.
- The solution of ionic compounds in water conduct electricity. This happens because ions present in the solution of an ionic compound facilitate the passage of electricity by moving toward opposite electrodes.
- Ionic compounds conduct electricity in the molten state.
Incorrect
Solution: (b)
Explanation:
- Ionic bonds are formed because of the transfer of electrons from the metal to the non-metal.
- In this course, metals get a positive charge because of the transfer of electrons and non-metal gets a negative charge because of acceptance of electrons. In other words, the bond formed between positive and negative ions is called Ionic Bond.
Since, a compound is electrically neutral, to form an ionic compound, negative and positive both ions must be combined. - The properties of an Ionic compound are:
- Ionic compounds have high melting and boiling points because the force of attraction between ions of ionic compounds is very strong. Hence statement 1 is incorrect.
- Ionic compounds are solid. Ionic bond has a greater force of attraction because of which ions attract each other strongly. This makes ionic compounds solid.
- Ionic compounds are brittle. Hence statement 2 is correct.
- Ionic compounds generally dissolve in water.
- Ionic compounds are generally insoluble in organic solvents; like kerosene, petrol, etc. Hence statement 3 is correct.
- Ionic compounds do not conduct electricity in the solid state.
- The solution of ionic compounds in water conduct electricity. This happens because ions present in the solution of an ionic compound facilitate the passage of electricity by moving toward opposite electrodes.
- Ionic compounds conduct electricity in the molten state.
-
Question 9 of 30
9. Question
Consider the following statements
- Calcination involves heating the sulphide ore in the absence of air to convert it into an oxide.
- Roasting involves heating the sulphide ore in the presence of air or oxygen..
Choose the correct code:
Correct
Solution: (b)
Explanation:
- Calcination is a process used for the decomposition of carbonate ores, not sulphide ores. Calcination involves heating the carbonate ore in the absence of air to convert it into an oxide. The purpose of calcination is to remove volatile impurities, such as carbon dioxide (CO2), and to facilitate the subsequent extraction of metals from the oxide ore. Hence statement 1 is incorrect
- During roasting, SO2 gas is released, and metal oxide is obtained.
- This statement is true. Roasting is a process used for sulphide ores. It involves heating the sulphide ore in the presence of air or oxygen. During roasting, the sulphide ore reacts with oxygen to form metal oxide and sulphur dioxide (SO2) gas. The metal oxide obtained is often easier to reduce or further process to extract the metal from the ore.Hence statement 2 is correct
Incorrect
Solution: (b)
Explanation:
- Calcination is a process used for the decomposition of carbonate ores, not sulphide ores. Calcination involves heating the carbonate ore in the absence of air to convert it into an oxide. The purpose of calcination is to remove volatile impurities, such as carbon dioxide (CO2), and to facilitate the subsequent extraction of metals from the oxide ore. Hence statement 1 is incorrect
- During roasting, SO2 gas is released, and metal oxide is obtained.
- This statement is true. Roasting is a process used for sulphide ores. It involves heating the sulphide ore in the presence of air or oxygen. During roasting, the sulphide ore reacts with oxygen to form metal oxide and sulphur dioxide (SO2) gas. The metal oxide obtained is often easier to reduce or further process to extract the metal from the ore.Hence statement 2 is correct
-
Question 10 of 30
10. Question
Consider the following statements
- Alloys are the homogeneous mixture of two or more metals only.
- Alloys are stronger than the metal from which they are obtained.
- The melting point of alloys can be lower than the constituent metals.
Which of the following statements is/are correct?
Correct
Solution: (c)
Explanation:
- Alloys are the homogeneous mixture of two or more metals, or a metal and a non-metal.
- Types of alloys:
- Ferrous alloys: An alloy in which iron (Fe) is present. For example : manganese steel (Fe = 86% ; Mn = 13% ; C = 1%) and Nickle steel (Fe = 98% ; Ni = 2%).
- Non-ferrous alloys: An alloy does not contain iron. For example : Brass (Cu = 80% ; Zn = 20%), and Bronze (Cu = 90% ; Sn = 10%).
- Amalgams: An alloy in which mercury (Hg) is present. For example Sodium amalgams [Na(Hg)] and Zinc amalgams [Zn(Hg)].
Hence statement 1 is incorrect.
- Alloys often exhibit improved mechanical properties compared to the pure metal components. By combining different metals or adding elements to the base metal, the alloy can enhance its strength, hardness, durability, or other desired properties. The presence of different elements in an alloy can alter the crystal structure, disperse stress, and hinder the movement of dislocations, resulting in improved strength and mechanical performance. Hence statement 2 is correct.
- The melting point of alloys can be lower than that of the constituent metals. When different metals are combined to form an alloy, the resulting mixture can have a different atomic arrangement and crystal structure compared to the pure metals. This altered structure can lead to a reduction in the melting point of the alloy. Example: Solder [Sn(80%) + Pb(50%)] has lower m. p. than Pb and Sn.
- The electrical conductivity of alloys is lower than that of constituent metals. Hence statement 3 is correct.
Incorrect
Solution: (c)
Explanation:
- Alloys are the homogeneous mixture of two or more metals, or a metal and a non-metal.
- Types of alloys:
- Ferrous alloys: An alloy in which iron (Fe) is present. For example : manganese steel (Fe = 86% ; Mn = 13% ; C = 1%) and Nickle steel (Fe = 98% ; Ni = 2%).
- Non-ferrous alloys: An alloy does not contain iron. For example : Brass (Cu = 80% ; Zn = 20%), and Bronze (Cu = 90% ; Sn = 10%).
- Amalgams: An alloy in which mercury (Hg) is present. For example Sodium amalgams [Na(Hg)] and Zinc amalgams [Zn(Hg)].
Hence statement 1 is incorrect.
- Alloys often exhibit improved mechanical properties compared to the pure metal components. By combining different metals or adding elements to the base metal, the alloy can enhance its strength, hardness, durability, or other desired properties. The presence of different elements in an alloy can alter the crystal structure, disperse stress, and hinder the movement of dislocations, resulting in improved strength and mechanical performance. Hence statement 2 is correct.
- The melting point of alloys can be lower than that of the constituent metals. When different metals are combined to form an alloy, the resulting mixture can have a different atomic arrangement and crystal structure compared to the pure metals. This altered structure can lead to a reduction in the melting point of the alloy. Example: Solder [Sn(80%) + Pb(50%)] has lower m. p. than Pb and Sn.
- The electrical conductivity of alloys is lower than that of constituent metals. Hence statement 3 is correct.
-
Question 11 of 30
11. Question
Consider the following statements
- Bases are sour in taste and turn blue litmus red.
- Acids are bitter in taste and turn red litmus blue.
Choose the correct code:
Correct
Solution: (d)
Explanation:
- Acids are sour in taste, turn blue litmus red, and dissolve in water to release H+ ions.
- Examples: Sulphuric acid (H2SO4), Acetic Acid (CH3COOH), Nitric Acid (HNO3), etc. Hence statement 1 is incorrect.
- Bases are bitter in taste, have soapy touch, turn red litmus blue, and give hydroxide ions (OH–) in an aqueous solution.
- Examples: Sodium hydroxide (caustic soda) – NaOH
Calcium hydroxide – Ca(OH)2
Hence statement 2 is incorrect.
Incorrect
Solution: (d)
Explanation:
- Acids are sour in taste, turn blue litmus red, and dissolve in water to release H+ ions.
- Examples: Sulphuric acid (H2SO4), Acetic Acid (CH3COOH), Nitric Acid (HNO3), etc. Hence statement 1 is incorrect.
- Bases are bitter in taste, have soapy touch, turn red litmus blue, and give hydroxide ions (OH–) in an aqueous solution.
- Examples: Sodium hydroxide (caustic soda) – NaOH
Calcium hydroxide – Ca(OH)2
Hence statement 2 is incorrect.
-
Question 12 of 30
12. Question
Consider the following
- China Rose Petals
- Litmus
- Onion
- Turmeric
- Cucumber
- Red cabbage
Which of the given above are commonly used natural indicators?
Correct
Solution: (b)
Explanation:
- Chia Rose Petals: China rose is indicator came from Hibiscus plant. China rose is a natural indicator. It turns an Acidic solution to dark pink / magenta colour.
- Litmus: Litmus is a natural indicator derived from lichens. It undergoes a color change in response to changes in acidity or alkalinity. Litmus turns red under acidic conditions and blue under alkaline conditions.
- Onion: Onion contains certain pigments that can act as natural indicators. When onion juice is extracted and used as an indicator, it can exhibit color changes in response to changes in pH.
- Turmeric: Turmeric is a spice derived from the root of the Curcuma longa plant. It contains a pigment called curcumin, which can act as a natural pH indicator. Turmeric turns yellow in acidic solutions and red or brown in alkaline solutions.
- Cucumber: Cucumber does not contain pigments that exhibit distinct color changes based on pH. Therefore, it is not commonly used as a natural indicator.
- Red cabbage: Red cabbage contains natural pigments called anthocyanins that can act as pH indicators. When red cabbage juice is used as an indicator, it changes color depending on the pH of the solution. It turns red in acidic solutions, purple in neutral solutions, and green or yellow in alkaline solutions.
Incorrect
Solution: (b)
Explanation:
- Chia Rose Petals: China rose is indicator came from Hibiscus plant. China rose is a natural indicator. It turns an Acidic solution to dark pink / magenta colour.
- Litmus: Litmus is a natural indicator derived from lichens. It undergoes a color change in response to changes in acidity or alkalinity. Litmus turns red under acidic conditions and blue under alkaline conditions.
- Onion: Onion contains certain pigments that can act as natural indicators. When onion juice is extracted and used as an indicator, it can exhibit color changes in response to changes in pH.
- Turmeric: Turmeric is a spice derived from the root of the Curcuma longa plant. It contains a pigment called curcumin, which can act as a natural pH indicator. Turmeric turns yellow in acidic solutions and red or brown in alkaline solutions.
- Cucumber: Cucumber does not contain pigments that exhibit distinct color changes based on pH. Therefore, it is not commonly used as a natural indicator.
- Red cabbage: Red cabbage contains natural pigments called anthocyanins that can act as pH indicators. When red cabbage juice is used as an indicator, it changes color depending on the pH of the solution. It turns red in acidic solutions, purple in neutral solutions, and green or yellow in alkaline solutions.
-
Question 13 of 30
13. Question
Consider the following statements
- Soaps are sodium or potassium salts of long-chain fatty acids.
- Detergents are ammonium and sulphonate salts of long-chain fatty acids.
- Soaps are non-biodegradable whereas detergents are biodegradable.
Which of the following statements is/are correct?
Correct
Solution: (a)
Explanation:
- Soaps are sodium or potassium salts of long-chain fatty acids.
- The ionic part of the soap is —COO–Na+.
Soaps efficiency decreases in hard water. Hence statement 1 is correct.
- Detergents are ammonium and sulphonate salts of long-chain fatty acids.
- The ionic part of the detergent is —OSO3-Na+.
- Detergent efficiency is unaffected in hard water. Hence statement 2 is correct.
- Soaps are biodegradable whereas detergents are non-biodegradable. Hence statement 3 is incorrect.
Incorrect
Solution: (a)
Explanation:
- Soaps are sodium or potassium salts of long-chain fatty acids.
- The ionic part of the soap is —COO–Na+.
Soaps efficiency decreases in hard water. Hence statement 1 is correct.
- Detergents are ammonium and sulphonate salts of long-chain fatty acids.
- The ionic part of the detergent is —OSO3-Na+.
- Detergent efficiency is unaffected in hard water. Hence statement 2 is correct.
- Soaps are biodegradable whereas detergents are non-biodegradable. Hence statement 3 is incorrect.
-
Question 14 of 30
14. Question
Consider the following statements
- Compounds of carbon which have only single bonds between the carbon atoms are called saturated compounds.
- Compounds of carbon which contain one or more double or triple bonds between carbon atoms are called unsaturated compounds.
Choose the correct code:
Correct
Solution: (c)
Explanation:
- Saturated compounds are organic compounds that contain carbon-carbon single bonds (C-C) and carbon-hydrogen single bonds (C-H). In saturated compounds, carbon atoms are bonded to the maximum number of hydrogen atoms possible, resulting in a stable and “saturated” configuration. Examples of saturated compounds include alkanes, such as methane (CH4), ethane (C2H6), and propane (C3H8).Hence statement 1 is correct.
- Unsaturated compounds are organic compounds that contain carbon-carbon double bonds (C=C) or carbon-carbon triple bonds (C≡C). Unsaturated compounds have fewer hydrogen atoms compared to saturated compounds due to the presence of multiple bonds between carbon atoms. Examples of unsaturated compounds include alkenes, such as ethene (C2H4) and propene (C3H6), which contain carbon-carbon double bonds, and alkynes, such as ethyne (C2H2), which contain carbon-carbon triple bonds. Examples: Ethene, Propene, Butyne, etc. Hence statement 2 is correct.
Incorrect
Solution: (c)
Explanation:
- Saturated compounds are organic compounds that contain carbon-carbon single bonds (C-C) and carbon-hydrogen single bonds (C-H). In saturated compounds, carbon atoms are bonded to the maximum number of hydrogen atoms possible, resulting in a stable and “saturated” configuration. Examples of saturated compounds include alkanes, such as methane (CH4), ethane (C2H6), and propane (C3H8).Hence statement 1 is correct.
- Unsaturated compounds are organic compounds that contain carbon-carbon double bonds (C=C) or carbon-carbon triple bonds (C≡C). Unsaturated compounds have fewer hydrogen atoms compared to saturated compounds due to the presence of multiple bonds between carbon atoms. Examples of unsaturated compounds include alkenes, such as ethene (C2H4) and propene (C3H6), which contain carbon-carbon double bonds, and alkynes, such as ethyne (C2H2), which contain carbon-carbon triple bonds. Examples: Ethene, Propene, Butyne, etc. Hence statement 2 is correct.
-
Question 15 of 30
15. Question
Consider the following statements
- A chemical reaction in which a substance burns in the presence of air or oxygen is called an reduction reaction.
- The addition of a molecule in unsaturated compounds in the presence of a catalyst is called an addition reaction.
Choose the correct code:
Correct
Solution: (b)
Explanation:
- A reduction reaction involves the gain of electrons or a decrease in oxidation state. Burning or combustion, on the other hand, involves the reaction of a substance with oxygen, typically resulting in the release of energy and an increase in oxidation state. Therefore, combustion is an example of an oxidation reaction, not a reduction reaction.Hence statement 1 is incorrect.
- Addition reactions involve the addition of atoms or groups of atoms to unsaturated compounds, such as alkenes or alkynes, resulting in the formation of new products. The addition can occur at the double or triple bond of the unsaturated compound, leading to the formation of a saturated compound. Catalysts are often used to facilitate the addition reaction by lowering the activation energy required for the reaction to proceed. Hence statement 2 is correct.
Incorrect
Solution: (b)
Explanation:
- A reduction reaction involves the gain of electrons or a decrease in oxidation state. Burning or combustion, on the other hand, involves the reaction of a substance with oxygen, typically resulting in the release of energy and an increase in oxidation state. Therefore, combustion is an example of an oxidation reaction, not a reduction reaction.Hence statement 1 is incorrect.
- Addition reactions involve the addition of atoms or groups of atoms to unsaturated compounds, such as alkenes or alkynes, resulting in the formation of new products. The addition can occur at the double or triple bond of the unsaturated compound, leading to the formation of a saturated compound. Catalysts are often used to facilitate the addition reaction by lowering the activation energy required for the reaction to proceed. Hence statement 2 is correct.
-
Question 16 of 30
16. Question
Consider the following statements
- The valency of an element is determined by the number of valence electrons present in the outermost shell of its atom.
- Atomic size is the distance between the centre of the nucleus and the outermost shell of an isolated atom.
- Electronegativity is the tendency of an element to attract the shared pair of electrons towards it in a covalently bonded molecule.
Which of the following statements is/are correct?
Correct
Solution: (d)
Explanation:
- The valency of an element is determined by the number of valence electrons present in the outermost shell of its atom (i.e. the combining capacity of an element is known as its valency).
- In Period: On moving from left to right in a period, the valency first increases from 1 to 4 and then decreases to zero (0).
- In Groups: On moving from top to bottom in a group, the valency remains the same because the number of valence electrons remains the same.
Example: Valency of first group elements = 1 Valency of second group elements = 2. Hence statement 1 is correct. - Atomic size refers to the radius of an atom. It is the distance between the centre of the nucleus and the outermost shell of an isolated atom.
- In Period: On moving from left to right in a period, atomic size decreases because nuclear charge increases.
Example: Size of second period elements: Li > Be > B > C > N > O > F - In Group: Atomic size increases down the group because new shells are being
added in spite of the increase in nuclear charge.
Example: Atomic size of first group element: Li < Na < K < Rb < Cs < Fr
Atomic size of 17th group elements : F < Cl < Br < I - The atomic size of noble gases in the corresponding period is the largest
due to the presence of a fully filled electronic configuration (i.e. complete octet). Hence statement 2 is correct. - Electronegativity is the tendency of an element to attract the shared pair of electrons towards it in a covalently bonded molecule. It increases with an increase in nuclear charge or a decrease in atomic size.
- Along the period electronegativity increases. Example: Li < Be < B < C < N < O < F.
- Down the group, electronegativity decreases. Example: Li > Na > K > Rb > Cs. Hence statement 3 is correct.
Incorrect
Solution: (d)
Explanation:
- The valency of an element is determined by the number of valence electrons present in the outermost shell of its atom (i.e. the combining capacity of an element is known as its valency).
- In Period: On moving from left to right in a period, the valency first increases from 1 to 4 and then decreases to zero (0).
- In Groups: On moving from top to bottom in a group, the valency remains the same because the number of valence electrons remains the same.
Example: Valency of first group elements = 1 Valency of second group elements = 2. Hence statement 1 is correct. - Atomic size refers to the radius of an atom. It is the distance between the centre of the nucleus and the outermost shell of an isolated atom.
- In Period: On moving from left to right in a period, atomic size decreases because nuclear charge increases.
Example: Size of second period elements: Li > Be > B > C > N > O > F - In Group: Atomic size increases down the group because new shells are being
added in spite of the increase in nuclear charge.
Example: Atomic size of first group element: Li < Na < K < Rb < Cs < Fr
Atomic size of 17th group elements : F < Cl < Br < I - The atomic size of noble gases in the corresponding period is the largest
due to the presence of a fully filled electronic configuration (i.e. complete octet). Hence statement 2 is correct. - Electronegativity is the tendency of an element to attract the shared pair of electrons towards it in a covalently bonded molecule. It increases with an increase in nuclear charge or a decrease in atomic size.
- Along the period electronegativity increases. Example: Li < Be < B < C < N < O < F.
- Down the group, electronegativity decreases. Example: Li > Na > K > Rb > Cs. Hence statement 3 is correct.
-
Question 17 of 30
17. Question
Consider the following statements
- Organic substances are more flammable and volatile whereas inorganic substances are generally non-volatile and inflammable.
- Organic substances are both soluble and insoluble in some organic solvents as well as in water whereas inorganic substances are insoluble in water.
- Organic substances contain carbon-hydrogen bonds whereas inorganic substances have no carbon-hydrogen bonds.
Which of the following statements is/are correct?
Correct
Solution: (c)
Explanation:
- Organic substances are more flammable and volatile whereas inorganic substances are non-volatile and inflammable.
- Fats, nucleic acids, carbohydrates, enzymes, proteins, and hydrocarbon fuels are examples of organic molecules.
- Non-metals, salts, metals, acids, bases, and things derived from a single element are examples of inorganic compounds. Hence statement 1 is correct.
- Most living organisms primarily consist of organic molecules whereas inorganic substances can be discovered in non-living things.
- Organic substances are often poor heat and electricity conductors in aqueous solutions.
- Inorganic substances are recognised as effective heat and electricity conductors in aqueous solutions.
- Organic substances are insoluble in water whereas inorganic substances are both soluble and insoluble in some organic solvents as well as in water. Hence statement 2 is incorrect.
- When it comes to organic substances, reaction rates are slow.
- When it comes to inorganic substances, reaction rates are comparatively high.
- Organic substances contain carbon-hydrogen bonds whereas inorganic substances have no carbon-hydrogen bonds. Hence statement 3 is correct.
Incorrect
Solution: (c)
Explanation:
- Organic substances are more flammable and volatile whereas inorganic substances are non-volatile and inflammable.
- Fats, nucleic acids, carbohydrates, enzymes, proteins, and hydrocarbon fuels are examples of organic molecules.
- Non-metals, salts, metals, acids, bases, and things derived from a single element are examples of inorganic compounds. Hence statement 1 is correct.
- Most living organisms primarily consist of organic molecules whereas inorganic substances can be discovered in non-living things.
- Organic substances are often poor heat and electricity conductors in aqueous solutions.
- Inorganic substances are recognised as effective heat and electricity conductors in aqueous solutions.
- Organic substances are insoluble in water whereas inorganic substances are both soluble and insoluble in some organic solvents as well as in water. Hence statement 2 is incorrect.
- When it comes to organic substances, reaction rates are slow.
- When it comes to inorganic substances, reaction rates are comparatively high.
- Organic substances contain carbon-hydrogen bonds whereas inorganic substances have no carbon-hydrogen bonds. Hence statement 3 is correct.
-
Question 18 of 30
18. Question
Consider the following statements
- Atoms of the same element having the same atomic number but different mass numbers are called Isobars.
- Atoms of different elements with different atomic numbers but the same mass numbers are called Isotopes.
- Any of two or more species of atoms or nuclei that have the same number of neutrons is called Isotone.
Choose the correct code:
Correct
Solution: (c)
Explanation:
- Atoms of the same element having the same atomic number but different mass numbers are called Isotopes.
- The atomic number of a particular atom is determined by the density of protons in that atom. The mass of protons in a chemical substance is constant.
- As a result, the atomic number of different atoms hailing from alike chemical substances is comparable to one another.
- Isotopes are thus atoms from the same chemical substance. Hence statement 1 is incorrect.
- Atoms of different elements with different atomic numbers but the same mass numbers are called Isobars.
- Isobars are referred to atoms with equal atomic masses from distinct chemical substances. The total of an atom’s neutrons and protons is known as its atomic mass. A nucleon is defined as a neutron or a proton. Isobars contain an equal amount of nucleons as one.
- Since various chemical components have a varying numbers of atoms, the atomic numbers of their isobars also differ.
- According to the Mattauch rule of isobars, if two neighbouring components from the periodic table contain isotopes with an alike mass number ( known as isobars), at least one isotope would be radioactive.
- Whenever three successive isobars occur, the first, as well as the final isobars, are generally stable, but the intermediate one might suffer radioactive decay.
- The isobar series seems to be a grouping of isotopes having identical atomic masses. Hence statement 2 is incorrect.
- Any of two or more species of atoms or nuclei that have the same number of neutrons is called Isotone.
- Thus, chlorine-37 and potassium-39 are isotones, because the nucleus of this species of chlorine consists of 17 protons and 20 neutrons, whereas the nucleus of this species of potassium contains 19 protons and 20 neutrons. Hence statement 3 is correct.
Incorrect
Solution: (c)
Explanation:
- Atoms of the same element having the same atomic number but different mass numbers are called Isotopes.
- The atomic number of a particular atom is determined by the density of protons in that atom. The mass of protons in a chemical substance is constant.
- As a result, the atomic number of different atoms hailing from alike chemical substances is comparable to one another.
- Isotopes are thus atoms from the same chemical substance. Hence statement 1 is incorrect.
- Atoms of different elements with different atomic numbers but the same mass numbers are called Isobars.
- Isobars are referred to atoms with equal atomic masses from distinct chemical substances. The total of an atom’s neutrons and protons is known as its atomic mass. A nucleon is defined as a neutron or a proton. Isobars contain an equal amount of nucleons as one.
- Since various chemical components have a varying numbers of atoms, the atomic numbers of their isobars also differ.
- According to the Mattauch rule of isobars, if two neighbouring components from the periodic table contain isotopes with an alike mass number ( known as isobars), at least one isotope would be radioactive.
- Whenever three successive isobars occur, the first, as well as the final isobars, are generally stable, but the intermediate one might suffer radioactive decay.
- The isobar series seems to be a grouping of isotopes having identical atomic masses. Hence statement 2 is incorrect.
- Any of two or more species of atoms or nuclei that have the same number of neutrons is called Isotone.
- Thus, chlorine-37 and potassium-39 are isotones, because the nucleus of this species of chlorine consists of 17 protons and 20 neutrons, whereas the nucleus of this species of potassium contains 19 protons and 20 neutrons. Hence statement 3 is correct.
-
Question 19 of 30
19. Question
Consider the following statements about the Nobel Prize in Chemistry 2022
- It was awarded to Benjamin List and David MacMillan.
- It was awarded for the development of Click Chemistry and Bioorthogonal Chemistry.
Choose the correct code:
Correct
Solution: (b)
Explanation:
- The Nobel Prize in Chemistry 2022 was awarded to Carolyn R Bertozzi, Morten Meldal and K Barry Sharpless.
- Sharpless came up with the term ‘click chemistry’ and worked extensively on it.
- Meldal, independently of Sharpless, came up with a special chemical structure called ‘triazole’ which has many significant applications.
- Bertozzi took the next step of developing click reactions that could work inside living organisms — ‘bioorthogonal’ reactions (a term she coined).
- The 2021 Nobel Prize in Chemistry was awarded to Benjamin List and David MacMillan for the development of asymmetric organocatalysis. Hence statement 1 is incorrect.
- It was awarded for the development of Click Chemistry and Bioorthogonal Chemistry.
- Click Chemistry is a minimalistic form of chemistry in which molecular building blocks can quickly and efficiently snap together. It is a form of simple and reliable chemistry, where reactions occur quickly, and unwanted by-products are avoided.
- Bioorthogonal Reactions work inside living organisms without disrupting the normal chemistry of the cell.
- Its use in combination with nanotechnology can lead to further developments in diverse areas of biomedicine, such as molecular bioimaging, targeted delivery, in situ drug activation, the study of cell–nanomaterial interactions, biosensing, etc. Hence statement 2 is correct.
Incorrect
Solution: (b)
Explanation:
- The Nobel Prize in Chemistry 2022 was awarded to Carolyn R Bertozzi, Morten Meldal and K Barry Sharpless.
- Sharpless came up with the term ‘click chemistry’ and worked extensively on it.
- Meldal, independently of Sharpless, came up with a special chemical structure called ‘triazole’ which has many significant applications.
- Bertozzi took the next step of developing click reactions that could work inside living organisms — ‘bioorthogonal’ reactions (a term she coined).
- The 2021 Nobel Prize in Chemistry was awarded to Benjamin List and David MacMillan for the development of asymmetric organocatalysis. Hence statement 1 is incorrect.
- It was awarded for the development of Click Chemistry and Bioorthogonal Chemistry.
- Click Chemistry is a minimalistic form of chemistry in which molecular building blocks can quickly and efficiently snap together. It is a form of simple and reliable chemistry, where reactions occur quickly, and unwanted by-products are avoided.
- Bioorthogonal Reactions work inside living organisms without disrupting the normal chemistry of the cell.
- Its use in combination with nanotechnology can lead to further developments in diverse areas of biomedicine, such as molecular bioimaging, targeted delivery, in situ drug activation, the study of cell–nanomaterial interactions, biosensing, etc. Hence statement 2 is correct.
-
Question 20 of 30
20. Question
Consider the following statements about Benzene
- It is a colourless liquid which is soluble in water.
- It has a sweet odour and is highly flammable.
- It is formed from both natural processes and human activities.
Which of the following statements is/are correct?
Correct
Solution: (b)
Explanation:
- Benzene is a colourless liquid which is insoluble in water.
- Benzene itself is a good solvent for many organic and inorganic substances e.g., fat, resins, sulphur and iodine. Hence statement 1 is incorrect.
- It has a sweet odour and is highly flammable.
- It bums with a luminous, sooty flame in contrast to alkanes and alkenes which usually bum with a bluish flame. Hence statement 2 is correct.
- It is formed from both natural processes and human activities.
- Natural sources of benzene include volcanoes and forest fires. Benzene is also a natural part of crude oil, gasoline, and cigarette smoke. Normal environmental concentrations of benzene are unlikely to damage animals or plants. It does have a low to moderate toxicity for aquatic organisms, but this is only likely to be apparent when high concentrations arise from significant spills.
- Fuels such as coal, wood, gas, kerosene or liquid petroleum gas (LPG) for space heating and cooking produce benzene through human activities. Hence statement 3 is correct.
Incorrect
Solution: (b)
Explanation:
- Benzene is a colourless liquid which is insoluble in water.
- Benzene itself is a good solvent for many organic and inorganic substances e.g., fat, resins, sulphur and iodine. Hence statement 1 is incorrect.
- It has a sweet odour and is highly flammable.
- It bums with a luminous, sooty flame in contrast to alkanes and alkenes which usually bum with a bluish flame. Hence statement 2 is correct.
- It is formed from both natural processes and human activities.
- Natural sources of benzene include volcanoes and forest fires. Benzene is also a natural part of crude oil, gasoline, and cigarette smoke. Normal environmental concentrations of benzene are unlikely to damage animals or plants. It does have a low to moderate toxicity for aquatic organisms, but this is only likely to be apparent when high concentrations arise from significant spills.
- Fuels such as coal, wood, gas, kerosene or liquid petroleum gas (LPG) for space heating and cooking produce benzene through human activities. Hence statement 3 is correct.
-
Question 21 of 30
21. Question
With reference to ‘Neutrinos’, consider the following statements
- Neutrino is a fermion that interacts only via the weak interaction and gravity.
- Neutrinos are the most abundant particles in the universe
- For each neutrino there also exists a corresponding antiparticle, called an antineutrino with opposite charge.
Choose the correct answer using the code given below
Correct
Solution (b)
Explanation:
- A Neutrino is a fermion (an elementary particle with spin of 1 / 2 ) that interacts only via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small (-ino) that it was long thought to be zero. Hence statement 1 is correct.
- Neutrinos are the second most abundant particles in the cosmos, produced in copious amounts in the cores of stars. Neutrinos are the second-most abundant particle in the universe after light particles (photons). Hence statement 2 is not correct.
- For each neutrino, there also exists a corresponding antiparticle, called an antineutrino, which also has spin of 1 / 2 and no electric charge. Antineutrinos are distinguished from neutrinos by having opposite-signed lepton number and weak isospin, and right-handed instead of left-handed chirality. Hence statement 3 is not correct.
Source: CLICK HERE
Incorrect
Solution (b)
Explanation:
- A Neutrino is a fermion (an elementary particle with spin of 1 / 2 ) that interacts only via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small (-ino) that it was long thought to be zero. Hence statement 1 is correct.
- Neutrinos are the second most abundant particles in the cosmos, produced in copious amounts in the cores of stars. Neutrinos are the second-most abundant particle in the universe after light particles (photons). Hence statement 2 is not correct.
- For each neutrino, there also exists a corresponding antiparticle, called an antineutrino, which also has spin of 1 / 2 and no electric charge. Antineutrinos are distinguished from neutrinos by having opposite-signed lepton number and weak isospin, and right-handed instead of left-handed chirality. Hence statement 3 is not correct.
Source: CLICK HERE
-
Question 22 of 30
22. Question
Consider the following statements about ‘Keeladi excavations’
- It is a Sangam period settlement lying on the bank of river Palar that is being excavated by the Archaeological Survey of India
- This site is estimated to be from the period between the 5th century BCE and the 3rd century CE and evidences of Tamil-Brahmi script have been found
Select the correct statement(s)
Correct
Solution (b)
Explanation:
- Keeladi excavation site is a Sangam period settlement that is being excavated by the Archaeological Survey of India and the Tamil Nadu Archaeology Department. The settlement lies on the bank of the Vaigai River. This is a large-scale excavation carried out in Tamil Nadu after the Adichanallur archaeological site. Hence statement 1 is not correct.
- In the fourth phase of excavations at Keezhadi, 72 potsherds with Tamil-Brahmi script were discovered at the site. Some of these artifacts have inscribed graffiti marks, similar to graffiti marks which some believe to have evolved from the Indus script. Hence statement 2 is correct.
Source: CLICK HERE
Incorrect
Solution (b)
Explanation:
- Keeladi excavation site is a Sangam period settlement that is being excavated by the Archaeological Survey of India and the Tamil Nadu Archaeology Department. The settlement lies on the bank of the Vaigai River. This is a large-scale excavation carried out in Tamil Nadu after the Adichanallur archaeological site. Hence statement 1 is not correct.
- In the fourth phase of excavations at Keezhadi, 72 potsherds with Tamil-Brahmi script were discovered at the site. Some of these artifacts have inscribed graffiti marks, similar to graffiti marks which some believe to have evolved from the Indus script. Hence statement 2 is correct.
Source: CLICK HERE
-
Question 23 of 30
23. Question
With reference to ‘Astrosat’, consider the following statements
- It is the first dedicated Indian astronomy mission aimed at studying celestial sources in X-ray, optical and UV spectral bands simultaneously
- The mission aims to understand high energy processes in binary star systems containing neutron stars and black holes and study star birth regions lying beyond our galaxy
Select the correct statement(s)
Correct
Solution (c)
Explanation:
AstroSat is the first dedicated Indian astronomy mission aimed at studying celestial sources in X-ray, optical and UV spectral bands simultaneously. One of the unique features of AstroSat mission is that it enables the simultaneous multi-wavelength observations of various astronomical objects with a single satellite. Hence statement 1 is correct.
The objectives of the mission are – To understand high energy processes in binary star systems containing neutron stars and black holes, estimate magnetic fields of neutron stars, study star birth regions and high energy processes in star systems lying beyond our galaxy, detect new briefly bright X-ray sources in the sky, perform a limited deep field survey of the Universe in the Ultraviolet region. Hence statement 2 is correct.
Source: CLICK HERE
Incorrect
Solution (c)
Explanation:
AstroSat is the first dedicated Indian astronomy mission aimed at studying celestial sources in X-ray, optical and UV spectral bands simultaneously. One of the unique features of AstroSat mission is that it enables the simultaneous multi-wavelength observations of various astronomical objects with a single satellite. Hence statement 1 is correct.
The objectives of the mission are – To understand high energy processes in binary star systems containing neutron stars and black holes, estimate magnetic fields of neutron stars, study star birth regions and high energy processes in star systems lying beyond our galaxy, detect new briefly bright X-ray sources in the sky, perform a limited deep field survey of the Universe in the Ultraviolet region. Hence statement 2 is correct.
Source: CLICK HERE
-
Question 24 of 30
24. Question
With reference to ‘Mulethi’, consider the following statements
- It is a fungus grown in the foothills of Himalayas
- It is endemic to India
- It has a sweet taste due to the presence of glycyrrhizin
- Himachal Pradesh became the first state to have organized cultivation of Mulethi
- India is one of the top exporters of Mulethi
Choose the correct answer using the code given below
Correct
Solution (d)
Explanation:
- It is a perennial herb grown in temperate zones. The plant parts used are runners and roots, which are collected in the fall season. Mulethi is the common name of Glycyrrhiza glabra, a flowering plant of the bean family Fabaceae, from the root of which a sweet, aromatic flavouring can be extracted. Hence statement 1 is not correct.
- The liquorice plant is an herbaceous perennial legume native to Western Asia, North Africa, and Southern Europe. Hence statement 2 is not correct.
- The roots have a sweet taste due to the presence of glycyrrhizin, which is 50 times sweeter than sucrose. Hence statement 3 is correct.
- Himachal Pradesh has recently begun the commercial cultivation of licorice (Mulethi) to become the first state in India to have organized cultivation of Mulethi. Hence statement 4 is correct.
- Mulethi is grown mainly in Afghanistan, while minor producing countries include Pakistan, China, Nepal and India. India imports 8047 tonnes of liquorice annually from Afghanistan. Hence statement 5 is not correct.
Source: CLICK HERE
Incorrect
Solution (d)
Explanation:
- It is a perennial herb grown in temperate zones. The plant parts used are runners and roots, which are collected in the fall season. Mulethi is the common name of Glycyrrhiza glabra, a flowering plant of the bean family Fabaceae, from the root of which a sweet, aromatic flavouring can be extracted. Hence statement 1 is not correct.
- The liquorice plant is an herbaceous perennial legume native to Western Asia, North Africa, and Southern Europe. Hence statement 2 is not correct.
- The roots have a sweet taste due to the presence of glycyrrhizin, which is 50 times sweeter than sucrose. Hence statement 3 is correct.
- Himachal Pradesh has recently begun the commercial cultivation of licorice (Mulethi) to become the first state in India to have organized cultivation of Mulethi. Hence statement 4 is correct.
- Mulethi is grown mainly in Afghanistan, while minor producing countries include Pakistan, China, Nepal and India. India imports 8047 tonnes of liquorice annually from Afghanistan. Hence statement 5 is not correct.
Source: CLICK HERE
-
Question 25 of 30
25. Question
ADT 52’ a term seen in news recently refers to
Correct
Solution (a)
Explanation:
ADT 52, which stands for ‘Aduthurai 52’, is a long-duration paddy variety. It takes at least 150 days for the plant to grow. It gives a high yield and can withstand the impact of unseasonal rains. It is resistant to gall midge and moderately resistant to leaf blast, neck blast, grain discoloration, and bacterial leaf blight.
Source: CLICK HERE
Incorrect
Solution (a)
Explanation:
ADT 52, which stands for ‘Aduthurai 52’, is a long-duration paddy variety. It takes at least 150 days for the plant to grow. It gives a high yield and can withstand the impact of unseasonal rains. It is resistant to gall midge and moderately resistant to leaf blast, neck blast, grain discoloration, and bacterial leaf blight.
Source: CLICK HERE
-
Question 26 of 30
26. Question
There is a statement in the following question after which there are two conclusions i.e. 1 and 2. You have to assume that the two statements are true and then choose the conclusion(s) that logically follows these statements, regardless of whether they are commonly known statements.
Statement: Most CPUs are keyboards. No keyboard is a Mouse. All Mouses are CPU.
Conclusion 1: Some keyboards are CPU
Conclusion 2: All CPU’s are Mouse
Conclusion 3: No Mouse is a keyboard
Conclusion 4: Some Mouse are keyboard
Which one of the following is correct?
Correct
Solution (b)
Explanation:
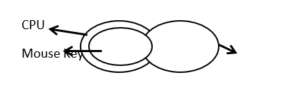
Clearly from the diagram conclusions 1 and 3 follow.
Incorrect
Solution (b)
Explanation:

Clearly from the diagram conclusions 1 and 3 follow.
-
Question 27 of 30
27. Question
There is a statement in the following question after which there are four conclusions. You have to assume that the statements are true and then choose the conclusion(s) that logically follows these statements, regardless of whether they are commonly known statements.
Statements:
I: Some Cats are Rats.
II: All bats are tables.
III: All Rats are Bats.
Conclusions:
I: Some Cats are bats
II: All bats are rats
III: All tables are cats
IV: All bats are cats
Choose the correct code:
Correct
Solution (d)
Explanation:
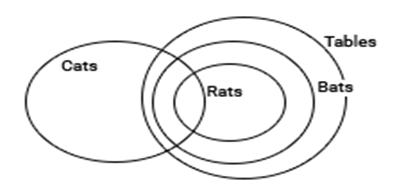
Clearly from the above Venn diagram only conclusion 1 follows
Directions for the following 2 (two) questions:
In each of the following questions one statement is given followed by two conclusions numbered 1 and 2. You have to take the given statement to be true even if it seem to be at variance from commonly known facts. Read the conclusions and then decide which of the given conclusions logically follows from the given statements.
Incorrect
Solution (d)
Explanation:

Clearly from the above Venn diagram only conclusion 1 follows
Directions for the following 2 (two) questions:
In each of the following questions one statement is given followed by two conclusions numbered 1 and 2. You have to take the given statement to be true even if it seem to be at variance from commonly known facts. Read the conclusions and then decide which of the given conclusions logically follows from the given statements.
-
Question 28 of 30
28. Question
Statement: Good voice is a natural gift but one has to keep practising to improve and excel well in the field of music.
Conclusions:
Natural gifts need nurturing and care
Even though your voice is not good, one can keep practising
Choose the correct code from below:
Correct
Solution (a)
Explanation:
I conclusion follows the statement, but second is not stated in statement so it does not follow
Incorrect
Solution (a)
Explanation:
I conclusion follows the statement, but second is not stated in statement so it does not follow
-
Question 29 of 30
29. Question
Statement: All the organised persons find time for rest. Anjana, in-spite of her busy schedule, finds time for rest
Conclusions
Anjana is an organised person
Anjana is an industrious person
Choose the correct code from below:
Correct
Solution (c)
Explanation:
Anjana has a busy schedule. This means that she is industrious. But still she finds the time for rest. This means that she is an organised person.
So both conclusions follow
Incorrect
Solution (c)
Explanation:
Anjana has a busy schedule. This means that she is industrious. But still she finds the time for rest. This means that she is an organised person.
So both conclusions follow
-
Question 30 of 30
30. Question
Read the following passage and answer the item that follow. Your answer to these items should be based on the passages only
Passage 1
Many sociologists have argued that there is functional relationship between education and economic system. They point to the fact that mass formal education began in industrial society. They note that the expansion of the economies of industrial societies is accompanied by a corresponding expansion of their educational systems. They explain this correspondence in terms of the needs of industry for skilled and trained manpower, needs which are met by the educational system. Thus, the provision of mass elementary education in Britain in 1870 can be seen as a response to the needs of industry for a literate and numerate workforce at a time when industrial processes were becoming more complex and the demand for technical skills was steadily growing.
The industry needs a literate work-force because
Correct
Solution (d)
Explanation:
Refer to
“Thus, the provision of mass elementary education in Britain in 1870 can be seen as a response to the needs of industry for a literate and numerate workforce at a time when industrial processes were becoming more complex and the demand for technical skills was steadily growing”…..
From this we can infer that option d is the correct answer.
Incorrect
Solution (d)
Explanation:
Refer to
“Thus, the provision of mass elementary education in Britain in 1870 can be seen as a response to the needs of industry for a literate and numerate workforce at a time when industrial processes were becoming more complex and the demand for technical skills was steadily growing”…..
From this we can infer that option d is the correct answer.
All the Best
IASbaba











