UPSC Articles
SalivaDirect test
Part of: GS Prelims and Mains II- Health issue
Context: On August 16, 2020, the United States Food and Drugs Administration authorised the emergency use of saliva based diagnostic test for COVID-19.About SalivaDirect test.
About SalivaDirect test
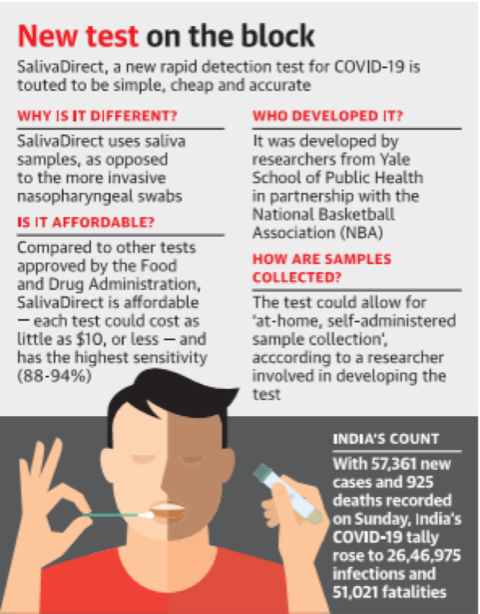
- SalivaDirect test is an inexpensive test, developed by a team from the Yale School of Public Health, has high sensitivity and can detect the virus even when the number of virus copies in the saliva sample is as low as 612 copies per microlitre.
- Collecting and testing saliva samples involves three steps — collecting saliva without preservative buffers, proteinase K treatment and heat inactivation, and dualplex RTqPCR virus detection.
- Disadvantages in previously using Nasopharyngeal swabs is it leads to false negative results due to errors at the time of sample collection.
- The sensitivity of the new SalivaDirect test was about 93%, according to a preprint posted on medRxiv “Official data shows 88-94% [sensitivity]” which is the best accuracy rate( sensitivity) of any saliva test as tweeted by Andy Slavitt, a former acting administrator of the Centres for Medicare and Medicaid Services in the Obama administration.
Pic source: The Hindhu











