Science and Technology
Context: Malaria is a life threating disease which kills nearly 600,000 people every year and the majority of whom are children under the age of five in sub-Saharan Africa.
- There is need to develop an effective vaccine against the disease with top priority — but given the highly complex life cycle of the parasite, characterization of key elements that correlate with protective immunity lead to difficulty in its development.
About Malaria:
- Malaria is a life-threatening disease caused by Plasmodium parasites, which are usually transmitted due to the bite of the female Anopheles mosquito
- These parasites swiftly multiply in the liver after being introduced in the host body, and destroy the red blood cells, thereby infecting the system
- Types of malaria: Malaria is caused by the bite of the female Anopheles mosquito, if the mosquito itself is infected with a malarial parasite.
There are five kinds of malarial parasites:
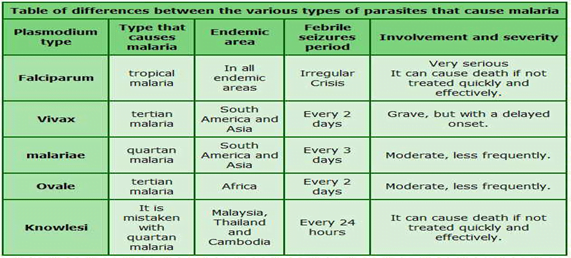
- Plasmodium falciparum
- Plasmodium vivax (the commonest ones)
- Plasmodium malariae
- Plasmodium ovale
- Plasmodium knowlesi.
- Therefore, if someone has contracted the Plasmodium ovale type of malaria means that the person has been infected by that particular parasite.
About Plasmodium ovale:
- P ovale is very similar to P vivax, which is not a killer form
- Symptoms include fever for 48 hours , headache and nausea, and the treatment modality is the same as it is for a person infected with P vivax.
- P ovale is no more dangerous than getting a viral infection
- P ovale malaria is endemic to tropical Western Africa.
Recent development in Vaccine development of Malaria:
- October 2021, WHO approved malaria vaccine RTS,S/AS01 (Mosquirix) developed by GlaxoSmithKline for immunizing children which is a major milestone.
- Although RTS, S/AS01 has modest efficacy and reduces severe malaria cases by only about 30 percent after four doses given to children under age 5, it still provides significant public health benefits, and could save thousands of lives every year.
- It took more than 30 years and approximately $700 million for this breakthrough, which underscores the scientific and logistic challenges in developing a vaccine against a parasitic disease like malaria.
- GSK has granted Bharat Biotech licence to manufacture Mosquirix, and by 2029, the Hyderabad-based company is expected to be the sole global manufacturer of this vaccine.
- However, RTS,S/AS01 fails to meet the WHO’s own benchmark for malaria vaccine efficacy of 75 per cent set in 2015.
- In September 2021, another malaria vaccine, R21/Matrix M, developed by the University of Oxford in the UK, demonstrated an efficacy of 77 per cent in phase 1 and 2 trials among 450 children in Burkina Faso.
- In early September 2022, this vaccine once again made headlines after publication of results of a booster dose of R21/Matrix-M in the journal Lancet Infectious Diseases showed a high efficacy of 80 per cent was maintained after two years.
Working of Vaccines:
- RTS,S and R21 are similar in that they both contain the same part of a major protein that is found on the surface of the liver stage parasite, called sporozoite.
- Both also contain hepatitis B virus surface antigen (HBsAg), a protein that has an ability to self-assemble and that helps as the formation of virus-like particles of the CSP antigen fused with it.
- The important difference between the two vaccines is in the amount of the HBsAg. RTS, S has about 20 per cent of the fusion protein, with the remaining 80 per cent made up of HBsAg antigen, produced separately.
- R21, on the other hand, is made up entirely of the CSP fusion protein moieties, resulting in much higher proportion of CSP antigen displayed on the virus-like particle surface, which significantly raises its exposure to the immune system of the host.
- RTS, S is formulated with an adjuvant called AS01 developed at GSK; R21 employs an adjuvant called Matrix-M developed by Novavax (Sweden).
- Matrix M contains saponin-plant based material and stimulates both antibody and cellular immune responses to vaccines.
India’s weakness and strength:
- Why has India not been more successful in developing vaccines against diseases including malaria — especially when basic malaria research in India has been robust and there are well established malaria research and control centres across the country.
- There is a major gap in the establishment of safe and scientifically robust control human infection models in India for diseases like malaria or influenza.
- All malaria vaccines under development need to be tested in the safe and scientific robust Controlled Human Malaria Infection (CHMI) model after completing phase 1 safety studies.
- This has been established in many countries of Europe, the UK, Colombia, and Thailand. Both RTS, S and R21 were tested in CHMI before further safety and efficacy field trials.
- Scientists at the International Centre for Genetic Engineering and Biotechnology (ICGEB) Delhi have carried out phase 1 safety trials of two experimental blood stage malaria vaccines developed and produced in the country.
- But further development of these vaccines has been a challenge in the absence of the CHMI model in India.
- With a highly successful and deeply committed vaccine-producing biopharma industry and a strong scientific base, India should be able to lead the world in developing and producing vaccines.
Indian government initiative to eliminate Malaria:
- Government has adopted the National Framework for Malaria Elimination (NFME) 2016-2030 outlines India’s strategy for elimination of the disease by 2030.
- The framework has been developed with a vision to eliminate malaria from the country and contribute to improved health and quality of life and alleviation of poverty.
Objectives of NSP:
- Achieve universal coverage of case detection and treatment services in endemic districts to ensure 100% parasitological diagnosis of all suspected malaria cases and complete treatment of all confirmed cases.
- Strengthen the surveillance system to detect, notify, investigate, classify and respond to all cases and foci in all districts to move towards malaria elimination.
- Achieve near universal coverage of population at risk of malaria with an appropriate vector control intervention.
- Achieve near universal coverage by appropriate BCC activities to improve knowledge, awareness and responsive behavior regarding effective preventive and curative interventions for malaria elimination.
- Provide effective programme management and coordination at all levels to deliver a combination of targeted interventions for malaria elimination.
Way Forward:
- “India has made remarkable progress in reducing the malaria incidence and deaths. Our efforts have resulted in 86.45% decline in malaria cases and 79.16% reduction in malaria related deaths in 2021 as compared to 2015.
- More than 124 districts in the country have reported ‘zero malaria case’’. This is a major step towards our goal for elimination of malaria but still more needs be done to fulfil the dream of Malaria Free India.
Source: Indian Express
Previous Year Question
Q.1) In the context of vaccines manufactured to prevent COVID-19 pandemic, consider the following statements:
- The Serum Institute of India produced COVID-19 vaccine named Covishield using mRNA platform.
- Sputnik V vaccine is manufactured using vector based platform.
- COVAXIN is an inactivated pathogen based vaccine.
Which of the statements given above are correct? (2022)
- 1 and 2 only
- 2 and 3 only
- 1 and 3 only
- 1, 2 and 3











