UPSC Articles
Medical Devices Notified as Drugs
Part of: GS Prelims and GS-II – Health
In News:
- With effect from 1st April, 2020, all Medical Devices shall be regulated by the Government as Drugs for quality control and price monitoring.
Key takeaways:
- The Maximum Retail Prices (MPRs) of all the Medical Devices would be monitored by the Government under the provisions of Para 20(1) of the Drugs (Prices Control) Order, 2013.
- This will ensure that no manufacturer/importer increases the MRP of a drug more than 10% of MRP during preceding 12 months, failing which penalty shall be imposed.
Important value additions:
- National Pharmaceutical Pricing Policy (NPPP) – governs price control.
- Drug Price Control Orders (DPCO) – enforces price control.
- National Pharmaceutical Pricing Authority (NPPA) – monitors and controls drug prices.
- National Pharmaceutical Pricing Policy-2012 – places a regulatory framework for pricing of drugs.
Miscellaneous
Azithromycin -Hydroxychloroquine Combination
- The Union Health Ministry has allowed the use of Hydroxychloroquine in combination with Azithromycin under severe conditions.
- The earlier guidelines which included use of Anti-HIV drugs Ritonavir-Lopinavir in high risk patients, now stand repealed.
- Azithromycin is commonly used as an antibiotic.
- Hydroxychloroquine (HCQ) is used in treatment of autoimmune diseases such as rheumatoid arthritis. It is also used in treating malaria.
- The azithromycin-hydroxychloroquine combination is part of an upcoming multi-country trial anchored by the World Health Organization to against COVID-19.
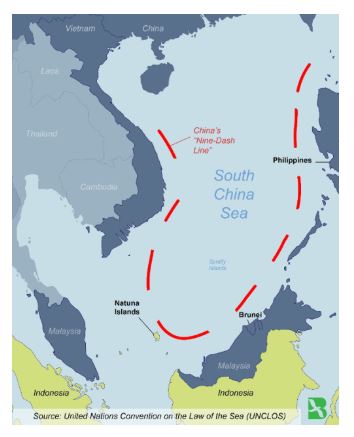
Natuna Islands
- Natuna Islands are within Indonesia’s exclusive economic zone but are also claimed by China.
- Recently, China has sparked a major maritime confrontation with Indonesia near the South China Sea by entering waters along with dozens of Chinese fishing vessels.
Medical Devices Notified as Drugs
Image source: Click here














